

Clinical Trials for
INDIVIDUALS WITH PHENYLKETONURIA (PKU)
NOW ENROLLING.
Jnana Therapeutics is committed to developing new therapies in areas of high unmet medical need.


Our team of scientists and clinicians have identified and are studying a new investigational medication called JNT-517 for the potential treatment of PKU.
Due to the challenge of adhering to the PKU diet and limited treatment options, some individuals with PKU have phenylalanine (Phe) levels that exceed the recommended range leading to symptoms such as difficulty with memory/thinking, depression, anxiety and poor quality of life. New therapies are needed that are safe, convenient, and allow for Phe reduction and increased natural protein intake.

Jnana’s Medication (JNT-517)
Will be taken by mouth
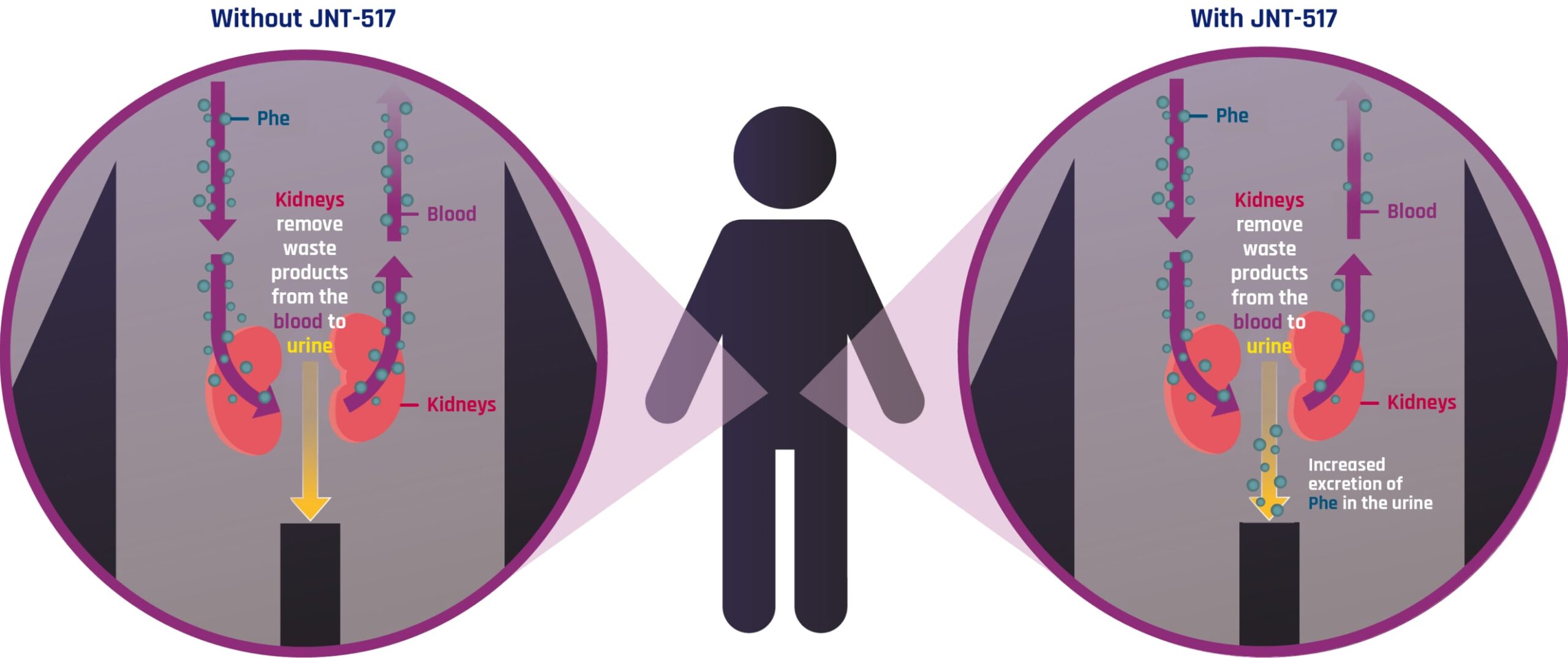
Designed to help the body get rid of excess Phe by:
Blocking Phe reabsorption from the kidney back into the blood
Increasing excretion of Phe in the urine
Studies of JNT-517
| Study | Phase | Description | Region | Study Status | Trial Number |
|---|---|---|---|---|---|
| JNT517-101 | Ib | Safety/Efficacy in healthy volunteers and PKU patients | US/AUS | Complete | NCT05781399 |
| JNT517-201 | II | Safety/Efficacy in adolescent PKU patients 12 to ≤18 | US/AUS | Now Enrolling Estimated Start Q1 2025 | NCT06637514 |
| JNT517-501 | III | Long Term Extension Safety/Efficacy in PKU patients | US/AUS | Now Enrolling Estimated Start Q1 2025 | NCT06628128 |
